So fresh water carrying O2 causes corrosion. How about a HOT WATER TANK feeder into the boiler?
So fresh water carrying O2 causes corrosion. How about a HOT WATER TANK feeder into the boiler?
Since the water is boiled already in the hot water tank, is this still counted as "FRESH WATER"?
Comments
-
Your water heater boils the water?
There is some thought that feeding a boiler with domestic hot water is helpful in this regard, but I'm not sure. I haven't tested at what temperature the oxygen is driven out of water.
I've been using distilled water for makeup water in my boiler (in addition to 8-way, a pH-booster) and I think my boiler might last 100 years as a result.
Edit: here's some data. I guess I will boil my water before I add it to my boiler now.
- Practical examples:
- Heating tap water to warm (50–60 °C) noticeably reduces dissolved oxygen but not completely.
- Bringing water to a rolling boil for a few minutes removes virtually all dissolved oxygen; this is why boiled water tastes flat and why deoxygenated water is used in some lab procedures requiring low O2.
- Short summary: dissolved oxygen gradually leaves as water is heated; boiling rapidly expels the remainder. At 1 atm, by the boiling point (~100 °C) the dissolved O2 concentration is essentially zero.
NJ Steam Homeowner.
Free NJ and remote steam advice: https://heatinghelp.com/find-a-contractor/detail/new-jersey-steam-help/
See my sight glass boiler videos: https://bit.ly/3sZW1el0 - Practical examples:
-
-
the water from my hot water tank is what feesd my steam boiler. im guessing since its already boiled and heated, theres no more o2?
0 -
What kind of hot water tank are you referring to that boils water?
NJ Steam Homeowner.
Free NJ and remote steam advice: https://heatinghelp.com/find-a-contractor/detail/new-jersey-steam-help/
See my sight glass boiler videos: https://bit.ly/3sZW1el0 -
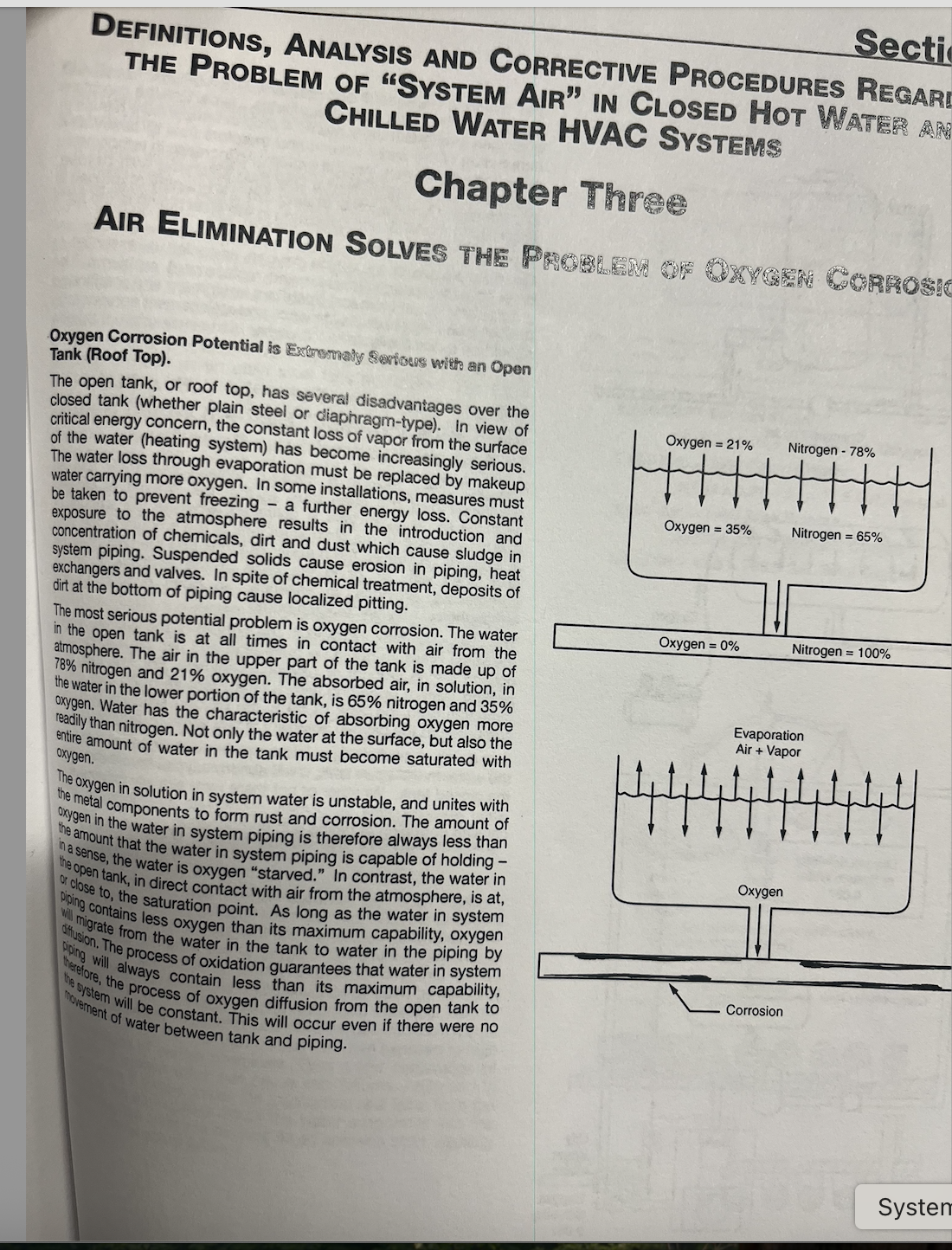
You are filling mostly with mostly nitrogen, their is approximately 20% O2 in H2O, 78% nitrogen.
The O2 will be consumed in days by the oxidation process of any ferrous metals, expansion tanks, pump bodies, etc. Rust never sleeps!
Unless additional O2 is entering somehow you should have "dead" O2 free water within a few days.
That being said, O2 has many ways to sneak in.
Mother nature hates all imbalances. If there is an O2 free condition, she will do everything to balance, so O2 goes into the system. The wall of plastic tube, even with an O2 barrier,
gaskets, seals, packings, etc.
Many installers add a hydronic conditioner which contains oxygen scavengers to handle small left over O2, or ongoing oxygen ingress.
Bob "hot rod" Rohr
trainer for Caleffi NA
Living the hydronic dream0 -
Commercial installations, especially when steam goes to waste, have "degas" devices to strip O2 & CO2 from feed water. Then there's complications concerning pumping that treated water while preventing air from re-entering.
0 -
An example of a large commercial deaeration device.
Rarely seen on residential systems.
Bob "hot rod" Rohr
trainer for Caleffi NA
Living the hydronic dream0 -
In process steam applications they sometimes preheat the water in a water heater to drive some of the dissolved air out and precipitate some of the minerals before putting it in to a steam boiler. If you aren't consuming the steam(or hot water), if it is a more or less closed system where the water returns to the boiler, there is little benefit to conditioning the water this way.
0 -
@hot rod has it exactly right. All large commercial and industrial steam systems should have Deaerators with boiler feed pumps to return water to the boiler. Also , the water should be treated as necessary by someone that knows what he is doing. This will extend the life of the boiler and piping system and allow the boiler to operate efficiently.
0 -
@hot_rod :
You are filling mostly with mostly nitrogen, their is approximately 20% O2 in H2O, 78% nitrogen.I think you are thinking of how much oxygen and nitrogen is in our air
The O2 will be consumed in days by the oxidation process of any ferrous metals, expansion tanks, pump bodies, etc. Rust never sleeps!
This is why the OP is asking about how to remove O2 from his makeup water. He just seems to be confused a bit and thinks that his water heater boils his water.
If you want to remove oxygen from your makeup water, just boil the water on your stove and pour it in your boiler.
NJ Steam Homeowner.
Free NJ and remote steam advice: https://heatinghelp.com/find-a-contractor/detail/new-jersey-steam-help/
See my sight glass boiler videos: https://bit.ly/3sZW1el0 -
You are filling mostly with mostly nitrogen, their is approximately 20% O2 in H2O, 78% nitrogen.I think you are thinking of how much oxygen and nitrogen is in our air
Yes and the air that is within the water.
Amtrol talks about the air, O-2 and nitrogen component. They did studies on the amount of "air" in water that enters you building.
This Amtrol Handbook should be required reading for anyone in the hydronic business. So, so many problems could be avoid if hydronic folk read this book. It is one of my most used books.
Excellent explanations of p
umping away, air removal, sizing, compression vs hydro pneumatic tanks, troubleshooting, etc.
I think an online version is out there.
Bob "hot rod" Rohr
trainer for Caleffi NA
Living the hydronic dream3 -
I am not in a position to counter how much nitrogen is in water, however, municipalities pump oxygen into the water they supply to homes to improve taste. So I will posit that the amount of oxygen in air is not a useful metric in determining how much oxygen is in household water.
NJ Steam Homeowner.
Free NJ and remote steam advice: https://heatinghelp.com/find-a-contractor/detail/new-jersey-steam-help/
See my sight glass boiler videos: https://bit.ly/3sZW1el0 -
Read the book and we can have a discussion. I trust Amtrol did their homework on this topic.
If you have a well or private source I don't think O2 is being added.
Public water providers do all sorts of things to water that might effect hydronic water.
I've visited several of the water providers in my state, and got tours of the their treatment facilities. I did not see or hear of O2 being added? That would have perked up my ears being in the plumbing and heating business, and specializing in air removal. But we have fairly good quality, good tasting Rocky Mountain water :)
Maybe back east it is about masking taste from all the forever chemicals that have made their way into aquifers :)
The amount of air in the water depends on the source, various pumping and treatments it goes through, how it is stored before it comes out of your tap, etc.
I doubt there is a set number to any of these observations and questions.
I think most would agree corrosion in open or closed systems has to do with the O2 inside, regardless of how or when it enters.
The DIN 4726 standard, if you can find and download a copy of that, talks a lot about this also, in regards to diffusion through tube wall
Bob "hot rod" Rohr
trainer for Caleffi NA
Living the hydronic dream1 -
Well, a water heater might help precipitate hard water depositing compounds ahead of the boiler, which seems like it could be helpful.
I wonder about chlorine and chloride sources. Peerless suggests keeping boiler charge below 30ppm chloride. How we supposed to do that? Anybody using carbon finishing filters?
Steward to 1923 Spanish revival near Chicago - 2 pipe steam 650 EDR shiny new Peerless 63-06
0 -
@EzzyT and @clammy install a filter on their feed water lines for sure. I don't think I've seen anyone else do it.
I use distilled water
NJ Steam Homeowner.
Free NJ and remote steam advice: https://heatinghelp.com/find-a-contractor/detail/new-jersey-steam-help/
See my sight glass boiler videos: https://bit.ly/3sZW1el0 -
The solubility of air and some salts in water decreases with temperature, you don't have to boil the water to drive a lot of the dissolved air out of the water. I'm pretty sure it is in something @DanHolohan wrote but if you heat water on the stove you start to see little bubbles forming on the surface of the pan and have a false hope that you are close to boiling at a much lower temp than boiling.
Municipalities do not add air to water to improve tase. They may add air for certain treatment processes to cause certain purification reactions to happen depending on what is in the raw water and what processes they use to remove it, but in general the water comes from sources with a lot of dissolved oxygen like lakes and rivers. These bodies of water are also usually fairly cold so the solubility of air in the water is higher. Air dissolved in municipal water sources isn't intentional, it is just a product of the physics of the processes. Since it is under pressure in the mains it keeps the dissolved air as it warms up. The weight of the water in the bodies of water it is pumped in also increases the pressure and the amount of dissolved air it is capable of holding.
0 -
These are becoming very common for one or two time use for filling residential systems.
These will scrub out all the + and _ ions giving you pure water. This will result in a low ph. The ph buffers up quickly as the water touches the metals in the system. The actual gallons you get out depends on how bad the water going in is.
Many, myself included, like to add a hydronic condition to give you even more protection . If the fill water is aggressive the conditioner buffers that.
A Culligan person told me much of the "distilled" water on store shelves is actually DI or DM water, maybe RO.
DI and DM is just running through a media, happens almost immediately.
I used to buy DI water from Culligan, they filled my 55 barrels with a 1" hose, that is how fast there equipment worked.
True distilled water is boiled, turned to steam, condensed on a stainless steel plate, so slower and more expensive to produce. Home counter top distillers for example produce about 1 gallon every 4-6 hours, using electricity. 500- 1500W depending on the model. No issue if you have PV I suppose :)
Very little difference between DI deionized, RO reverse osmosis, and distilled water, for boiler filling.
Those machines at Walmart are a pretty good deal for water in 5 gallon containers. I looked behind one and it looks like RO, a tank and some polishing filters, maybe carbon.
If you want 55 gallons call some local water treatment companies, Culligan, etc.
Bob "hot rod" Rohr
trainer for Caleffi NA
Living the hydronic dream0 -
Where ever there's a vent or vacuum breaker air can enter steam heating system. Lost Art describes air eliminators in lieu of vents. I've seen boilers enjoy regular chemical treatments (plus blow down top and bottom) but condensate lines still rot out without degas.
An unsolved mystery is how HHW consumes water and ingests air. Supposedly modern pump seals are blamed?
0 -
effectively the only difference between deionized and distilled is that deionized still has carbon dioxide in it forming carbonic acid.
0 -
DI also removes carbon dioxide from water? CO2 in the water contacts the ion exchange resin converts it into bicarbonate ions which are removed by the media.
With a CO2 imbalance, immediately that water will start to reabsorb CO2 from the atmosphere, so keep it contained and get it in quickly. That mother nature imbalance concept again.
Bob "hot rod" Rohr
trainer for Caleffi NA
Living the hydronic dream0 -
Thanks for this clarification, Matt. I must have mis-read something at some point and am grateful for the correction!
NJ Steam Homeowner.
Free NJ and remote steam advice: https://heatinghelp.com/find-a-contractor/detail/new-jersey-steam-help/
See my sight glass boiler videos: https://bit.ly/3sZW1el0 -
Cool, I will check them out. Of course i already have an RO system for drinking water, maybe I should just use that? It claims to do 75 gal per day, so even a full fill wouldn't be horrible. Without a bigger holding tank it might take 5 hours to fill
Steward to 1923 Spanish revival near Chicago - 2 pipe steam 650 EDR shiny new Peerless 63-06
0 -
@hot_rod said:
True distilled water is boiled, turned to steam, condensed on a stainless steel plate, so slower and more expensive to produce.
Or it's collected for free from your basement dehumidifier like mine is!
NJ Steam Homeowner.
Free NJ and remote steam advice: https://heatinghelp.com/find-a-contractor/detail/new-jersey-steam-help/
See my sight glass boiler videos: https://bit.ly/3sZW1el0 -
-
we were told that the di water in high school chemistry lab was acidic because it still had C02 combined with it. Don't remember if I ever tested that but I thought you saw it in some steps of the analysis process.
0 -
Early on @hot_rod said: "You are filling mostly with mostly nitrogen, their is approximately 20% O2 in H2O, 78% nitrogen."
I know that Bob Rohr, along with many others, knows this but the way it is written it may be misunderstood that the O part of H2O is being removed from the water. That is not the case. H2O is the chemical compound that is known as water (or ice or steam). Removing the O from that compound is a chemical reaction that will form free oxygen and free hydrogen atoms. That is not the case here for those of you that are new to the concept of dissolved gasses in water. Study Boyle's law for that explanation. The oxygen that is over 20% with the nitrogen that is about 78% along with the other trace gasses in the atmosphere are what Bob is referring to.
Those are the gasses that are dissolved in the water including that oxygen. That O2 will work on any ferrous metals in short order. As the dissolved gasses in the water become void of oxygen as it forms rust in the cast iron and steel parts of the boiler system, the ability of that water to continue to cause more rust vanishes. That is what we are trying to achieve in a hydronic system. Dissolved oxygen depleted water circulating thru the pipes will be inert and will be a great heat transfer medium for a hydronic system. Unless you have oxygen ingress thru non barrier tubing or air vent leaks or packing glands, etc… That water will stay oxygen free for as long as you leave it in the system.
This steam boiler/water heater pretreat discussion reminds me of a bakery that uses process steam to make a type of bread. When it was time to replace the boiler they went cheap on a boiler replacement. Since there was no return condensate to the steam boiler, the water that was being converted to steam was always fresh (full of dissolved oxygen) cold water. As a result, the boilers were failing in as little as 6 years. “What can we do about this?”
By adding a residential water heater and a SpiroVent before the boiler feed, heating the boiler feed water to 140°, the water contained much less dissolved gasses resulting in much less dissolved O2. The boiler would last much longer. Here is the article about it
Edward Young Retired
After you make that expensive repair and you still have the same problem, What will you check next?
2 -
well said Ed.
You would not believe how many homeowners I’ve met who were told by their installer to drain their boiler every two weeks to keep it clean. Or maybe you would believe it!
NJ Steam Homeowner.
Free NJ and remote steam advice: https://heatinghelp.com/find-a-contractor/detail/new-jersey-steam-help/
See my sight glass boiler videos: https://bit.ly/3sZW1el0
Categories
- All Categories
- 87.5K THE MAIN WALL
- 3.3K A-C, Heat Pumps & Refrigeration
- 61 Biomass
- 430 Carbon Monoxide Awareness
- 122 Chimneys & Flues
- 2.1K Domestic Hot Water
- 5.9K Gas Heating
- 118 Geothermal
- 170 Indoor-Air Quality
- 3.8K Oil Heating
- 78 Pipe Deterioration
- 1K Plumbing
- 6.6K Radiant Heating
- 395 Solar
- 15.9K Strictly Steam
- 3.5K Thermostats and Controls
- 57 Water Quality
- 51 Industry Classes
- 51 Job Opportunities
- 18 Recall Announcements