Discolored hot water / HTP PH 76-60 hot water heater installed 5 months ago

Hi! I'm just a homeowner looking for help! Five months ago we had an HTP Phoenix 76-60 stainless steel hot water heater installed, but only about a week ago we started seeing discolored hot water (pale yellow-green) when running a bath. Cold water was clear. We have a whole house sediment filter which was changed out five months ago, and a Hague water softener, which was set to less than 1 grain at the time…
We drained our hot water heater as best as we could by filling the tub a couple of times, hoping to flush out whatever was discoloring the water. The color faded, but returned the next day.
We Googled and saw that possibly lowering the temperature of our hot water heater could help, so we took it down from 140 to 126 degrees. We also cleaned our water softener beads with Iron Out and did a regen. Calling up HTP yielded the advice to add more grains of hardness to our softened water. Luckily our softener has a mixing valve, so we raised it up to 5 grains, their minimum hardness recommendation.
We called up our town's water department and they confirmed that there have been no recent maintenance events, and reassured me that pH and chloride tests are all within spec.
All of these initial changes didn't make a difference, so we ordered up many water testing kits. Here are our stats:
pH: >7.6 (as high as our test could go, but was told that our town has been testing at 7.7 for forty years)
COPPER: Softened cold water: 0.0 ppm / Softened hot water: 0.1 ppm
CHLORINE: Municipal tap: 0.3 ppm / Softened water: 0.05 ppm
IRON: Municipal tap: 0.3 ppm / Softened cold water: 0.12 ppm / Softened hot water: 0.6 ppm (double the municipal feed)
So we’re assuming the yellowish-green color is from iron and a little bit of copper somewhere in between the stainless steel hot water heater and our tap. But what could be causing this?
Calling HTP again, they swore that we must have some galvanized pipes somewhere in our system, causing the corrosion to the stainless steel. Our system is all copper. We do have some aluminum clamps with zinc screws, but they said that shouldn’t affect our water.
Another thing HTP guessed was that the soft water could be reacting with the cupronickel coil. Is this likely? How delicate are these stainless steel units?
What we don’t want is water harmful to bathe in, or corrosion to our hot water heater elements or tank, or our pipes!
Does anyone have any insights? @Larry Weingarten — I see you mentioned on this forum for good advice? I would be so grateful! Thanks in advance?
Comments
-
-
Well, the colour strongly suggests mostly copper, with a little iron possibly mixed in.
That said… I see you measured Chlorine — a water treatment disinfectant — but not Chloride, which is only one letter different but chemically very different. And, unfortunately, a byproduct of any type of ion exchange water softener, and can be very corrosive on some types of stainless steel, as well as copper pipes. That may be your problem.
Br. Jamie, osb
Building superintendent/caretaker, 7200 sq. ft. historic house museum with dependencies in New England0 -
depending on the model, some htp indirects have a copper nickel hx, that is probably dissolving. could be the over softened water, cold be some stray current in the plumbing or probably both.
100 ppm is htp's limit on chlorides, at least on my ~2019 superstore.
0 -
Hi, I would stop softening the hot water complely and see what difference it makes. Stainless and salt don't get along at all! I've seen recommendation from the National Association of Corrosion Engineers that you leave 60 to 120 ppm of calcium and magnesium hardness in the water after softening. Otherwise it can damage even copper pipe.
I'd be tempted to drain out the softened water and refill with unsoftened, to give you a good start on identifying the problem. 😉
Yours, Larry
1 -
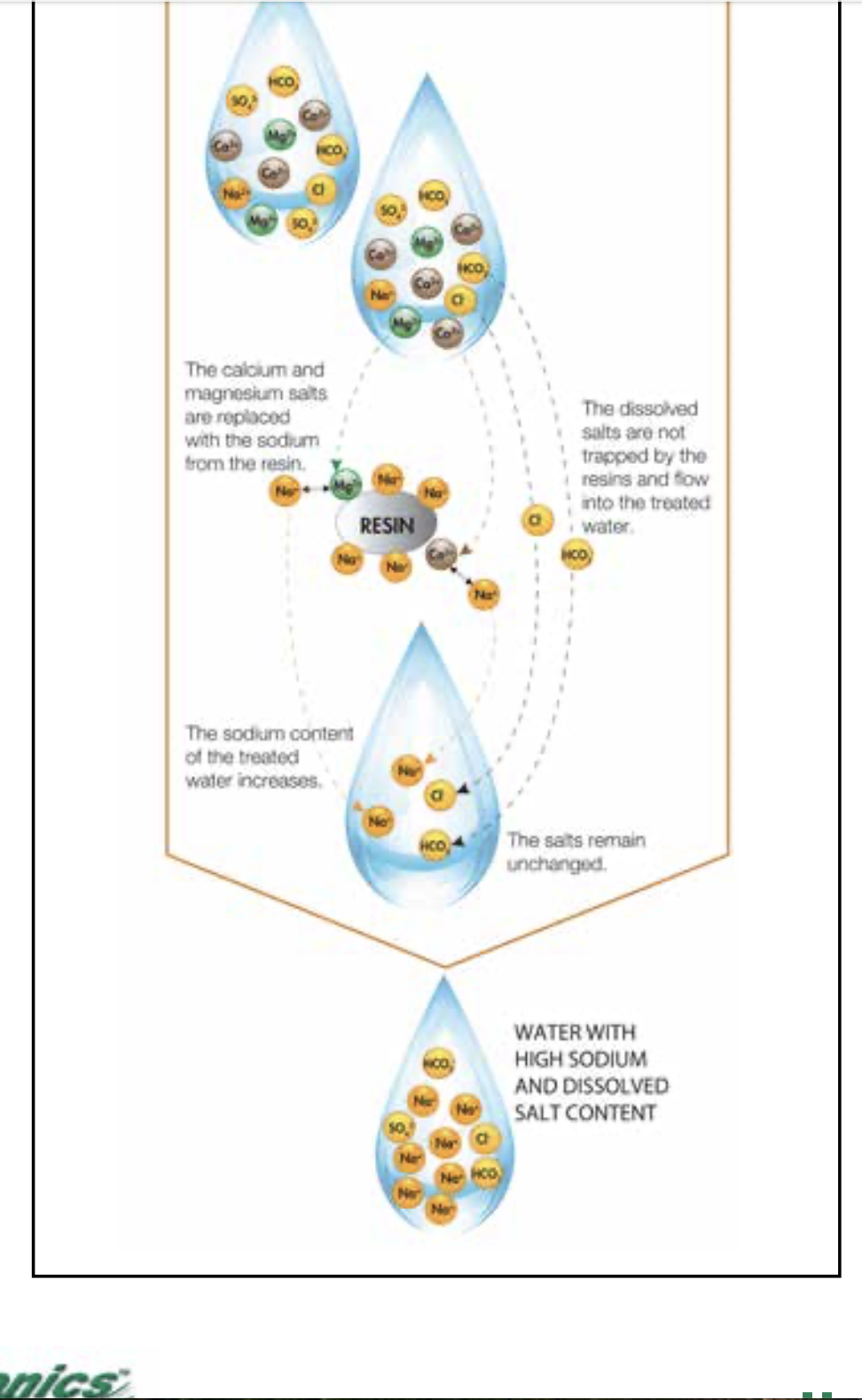
This is how an ion exchange water softener works.
Bob "hot rod" Rohr
trainer for Caleffi NA
Living the hydronic dream0 -
Hi! I have some updates! So, per your advice @Larry Weingarten, we drained our tank and bypassed our salt-based water softener. Iron levels in our hot water did drop from 0.6 ppm to 0.3-0.4 ppm, which matches our municipal tap, so obviously our softener is an issue. The color of our bath water did change as well, to a more pale blue color vs greenish, if that tells us something…
But we got our water softener for a reason — water in our town runs at 22 grains of hardness and we don’t want all of that scaling in our pipes and machines! We turned our softener back on to 7 grains this time, which is much harder than the <1 grain we were set at when we first saw the yellow-green water. We’re hoping this could be a happy medium of softened water that isn’t corroding our stainless steel HTP tank? Do you have further thoughts?
Also, I was wondering how long would it take for us to really see if these adjustments have made an effect?
@hot_rod, @Jamie Hall, @mattmia2 We also bought a Hanna chloride test kit and the fine test put us at >100 ppm, and the coarse test put us at 100 ppm, testing at 7 grains of hardness. This softened water matches the chlorides in our municipal feed, so apparently our softener isn’t elevating levels. HTP recommends a maximum chloride level of 100 ppm — could being slightly over actually be the culprit? HTP will keep blaming anything out of spec and suggests I work on getting chlorides lower. How would I do that? (Besides reverse osmosis, which we found brought our chlorides down to 10 ppm.)
We bought this HTP unit on the recommendation of our plumber, who said that it would be the most straight-forward to service. But now that we’ve learned that the stainless steel and cupronickel heating element can corrode under less-than-perfect circumstances, we’re wondering why we just didn’t get another glass-lined tank — at probably less than half of what this cost us!
0 -
Your chloride level is still too high for stainless steel in my opinion — if I had to guess, I would guess a road salt problem in your town's domestic supply, but there could be other reasons (yes, it's legal — the EPA max. is 250 mg/L at last count). Glass lined would have been better…
Br. Jamie, osb
Building superintendent/caretaker, 7200 sq. ft. historic house museum with dependencies in New England1 -
Hi, It sounds like what's good for the tank isn't good for you. I'd go with glass-lined when the time comes… and for some un-asked-for advice; I'd use only RO water for cooking and drinking. 😉
Yours, Larry
ps, When you have a new tank, use a powered anode in it. This won't degrade as anodes in very conductive water do.
1 -
I'm guessing it is the softened water and possibly other lingering effects from the softened water. I have an ssu45 and water with abut 120 ppm chloride and I don't have discolored water. Our water also has a lot of corrosion inhibitor in it.
0 -
@Jamie Hall, @Larry Weingarten, @mattmia2, thanks for the advice!
Running on 7 grains of hardness for days now, we still have a slight tinge of green to our water — definitely an improvement from where we started. I ran an online calculator and saw that 7 grains equals 120 ppm — Larry, would this satisfy the Corrosion Engineers? Do you think we'll be continuing to degrade our stainless steel tank / heating element? Do you think one day this green color might go away, after whatever was left in the tank flushes out and any new copper skins over?
How would one lower levels of chlorides in our water? Is there a way?
And, Matt, tell me more about corrosion inhibitor? Though I see your stainless tank doesn't have a heater…
Our plumber did a fastidious job and seemed like some kind of OCD savant, so we really trusted his advice — but maybe we shouldn't have re-invented the wheel. We previously had a regular old glass-lined Rheem that leaked all over our basement (official diagnosis was a blown PRV), but then got scared to replace it with the same unit because reviews are so bad online… What water heaters would you recommend for "next time?"
And, much longer do you think our HTP unit will last, in all honesty? Can we get 10 years out of this?
I really appreciate all of your help sorting this out! Our plumber and HTP are just not neutral parties to consult, and it's killing me that it seems we spent so much money on something not compatible with our system.
0 -
Sorry to say it, but… with your water quality, I'd go with a good glass lined gas fired tank… and the Rheem tanks are pretty good. On line reviews are a VERY poor guide to quality, by the way.
Br. Jamie, osb
Building superintendent/caretaker, 7200 sq. ft. historic house museum with dependencies in New England0 -
Oh, it is a phoenix, not an indirect of some sort. The higher temp probably means it is more reactive with the water than in the indirects.
Maybe try limiting the firing rate in the controls and see if that makes the problem go away and you still get enough hot water.
Also try really flushing it and wanding it through the drain to see if you can get all of the crud out of the bottom.
0 -
It looks like it has an anode, did you take a loo at that? could try a powered anode too. the anode can dissolve very quickly in oversoftened water.
0 -
@mattmia2 One of the first things we did was lower the temp to 126 degrees, as it was set up at 140… But thanks for the idea of wanding it — we drained the tank completely twice, but I didn't realize there could actually be crud down there, since the unit is fairly new.
Could a powered anode be installed in my Phoenix?
@Jamie Hall I'm coming to this forum first before I buy another unit!
0 -
-
Hi, A question. With the water testing that you've done you say TDS is at 120. Did the lab test separately for sodium, or is that includes in the TDS? If that number is primarily calcium and magnesium, it is good. If sodium is a large part, not good. … Green water color usually means copper. Did the lab test for copper? If the water is dissolving copper, something must change. If there is a place where a piece of copper could either be looked inside of, or cut out and looked at, it would be telling? If there is a nice thin coating of scale, great. If there is no coating and some erosion has taken place, not so good. 😕
Yours, Larry
0 -
One could probably stick a borescope in the anode tapping and look at the hx.
0 -
@Larry Weingarten Ohhhhh! I thought grains of hardness was the same thing as TDS! Maybe we do have to get our water sent out to be tested by a lab…. Our personal tests for copper were low, only the iron looked elevated, but I guess we are just laypeople.
@kcopp ActivFlo produces a galvanic reaction, right? That wouldn't be good for my stainless steel tank?
0 -
Besides everything else… I'm wondering what the pH of your softened water is. Not the source water, the softened water. Reason I'm wondering is, first, in some instances softening can lower the pH, depending somewhat on the exact chemistry of the water. Second, and more on the I wonder end, is that the greenish colour is often the result of mild — but continuing — corrosion of copper piping, which is increased by a low or lower pH…
Br. Jamie, osb
Building superintendent/caretaker, 7200 sq. ft. historic house museum with dependencies in New England1 -
Couple days, however long it takes to flush through what is in the tank. I suspect with your very conductive water that the problem is that the anode has dissolved.
A softener replaces the not very soluble ions like calcium and iron in the water with more soluble sodium ions so if there was a high number of ions in the water making it conductive those will still be in the water after the softener. The more current that flows the faster the anode dissolves.
0 -
The more "stuff" that is in you water the higher the conductivity. A basic VOM could show you this, test before and after the softener.
You can get pure water a few ways RO, generally just at the drinking water, kitchen sink for treating bad water.
DI or DM deionized or demineralized water, this uses a resin bed similar to a softener.
Distilling, boil water to steam, collect the condensate..
All 3 are fairly expensive for a whole house solution, Also, pure water is not so good for drinking, some minerals are good for you, give the water some taste. Pure water from one of the 3 methods will also be low ph. Until they pull some from metals in the system. It is hard to keep pure, hungry water pure for much time, ph will balance back to neutral.
As you see water can be a very complex product to adjust and get to where you want it. You really want a water treatment pro that can explain what you have, what you can do, and how to do it.
Unscrupulous water treatment people sell softeners, or RO or carbon block filters as a fix all, it is not always that simple and can make matter worse, depending on what is in your water.
Complicating it even more is the water can change over the course of the year depending on the source(s). Spring runoff in snowbelt areas may show high chloride levels for the deicers. That goes away or lowers as the roads flush clear.
Bob "hot rod" Rohr
trainer for Caleffi NA
Living the hydronic dream0 -
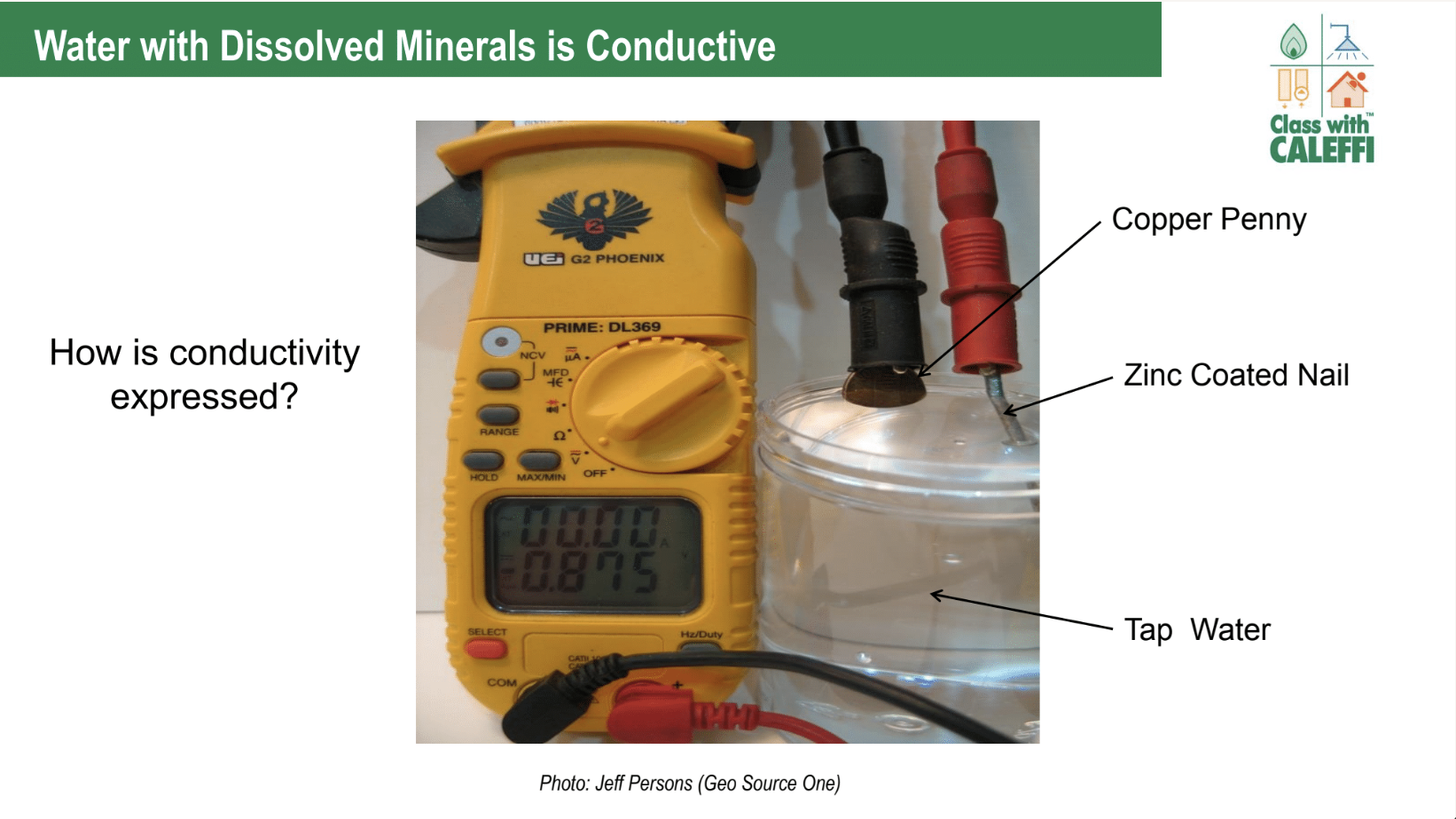
the penny and galvanized metal are a battery, you need the meter set to dc volts, not resistance. that isn't measuring resistance , it is measuring the voltage created by the battery but the meter is trying to interpret that voltage as the voltage drop in its resistance measuring circuit. to measure the resistance you need probes of the same metal. the actual resistance of the hard water s probably in the megaohm range and essentially infinity for the di water though that will depend on how much CO2 is dissolved in it.
1 -
Hi @barbap , Just to clarify. You said "I thought grains of hardness was the same thing as TDS!" … TDS means total dissolved solids. Hardness usually refers to calcium and magnesium, but sodium can be a dissolved solid as well. Particularly with softened water, where salt is exchanged for calcium and magnesium, it's important to measure sodium directly, so you know what's really in your water… if it's safe to drink … and if it's okay to use in stainless tanks and copper plumbing. High salt and low pH as @hot_rod brought up is a combination ultimately lethal to the metals used in plumbing.
Yours, Larry
0 -
-
oh, sorry. i thought there were differing electrodes in all 3. the resistance will depend on the area of the electrodes too. there is an old style of dummy load where iron plates are lowered in to a vat of brine.
0 -
@Jamie Hall So we have a pH test kit for an aquarium, and all of our water — municipal tap, softened water, and RO — tested to above 7.6 (where the test maxed out). Our town water guy told me that the town has been testing at 7.7 for the past 40 years, so we're assuming that our pH is okay…
0
Categories
- All Categories
- 87.5K THE MAIN WALL
- 3.3K A-C, Heat Pumps & Refrigeration
- 61 Biomass
- 430 Carbon Monoxide Awareness
- 123 Chimneys & Flues
- 2.1K Domestic Hot Water
- 5.9K Gas Heating
- 118 Geothermal
- 170 Indoor-Air Quality
- 3.8K Oil Heating
- 78 Pipe Deterioration
- 1K Plumbing
- 6.6K Radiant Heating
- 395 Solar
- 15.9K Strictly Steam
- 3.5K Thermostats and Controls
- 57 Water Quality
- 51 Industry Classes
- 51 Job Opportunities
- 18 Recall Announcements