Riello Burner on Biasi
Comments
-
If it's a B10 with an F5, then yes, the few that I've dealt with have been a little quirky using the spec settings. Where they ask for an 80°, I've put in 70°, or made drastic changes to the turbulator, air gate, and/or pump pressure to get what I want. I typically want 4.5%-6.5% O2 with 25% excess air. As long as everything else is within range.
What's the draft readings?
0 -
-
Biasi , they are a possitive pressure boiler ..
There was an error rendering this rich post.
0 -
You don't hear much of Biasi last few years...still in business? Mad
0 -
Higher pump pressures (over 140) with bio fuels tend to be an issue.
0 -
I never heard that. Nor have I seen that being a problem in the field.
Practically no set ups are with 100psi anymore.
Anyone else?There was an error rendering this rich post.
0 -
I was taught that higher pressure with a slightly smaller nozzle, maintaining the same firing rate and spray pattern, was a good way to get around fuel quality problems. It's always worked for me. When updating an older burner this way, I like to put a label on the burner with the nozzle spec and pump pressure used, so the next person (if it isn't me) knows.
All Steamed Up, Inc.
Towson, MD, USA
Steam, Vapor & Hot-Water Heating Specialists
Oil & Gas Burner Service
Consulting0 -
-
Really, If 1 pound of sugar is sweet 5 pounds is 5 times a sweet. Nevermind the concentration of bio when going into the call. I must be wrong then.
0 -
I'm not saying you're wrong. I'm saying I've never heard of that and was wondering if anyone else every heard of that. The conventional proven wisdom is that upping the pump pressure and reducing nozzle size gives better combustion.
There was an error rendering this rich post.
0 -
Cranking pump pressures and smaller nozzles are not always the answer. Tempermental Riello burnes, revert to specs from 25+ years ago.
Higher pump pressure and smaller nozzle size gives finer atomization not necessarily combustion.
0 -
I've got this great recipe for chocolate icing. Only uses 3 cups of sugar
- 1/2 cupbutter or margarine (1 stick)
- 2/3 cupHERSHEY'S Cocoa
- 3 cupspowdered sugar
- 1/3 cupmilk
1 tspvanilla extract
Edward Young Retired
After you make that expensive repair and you still have the same problem, What will you check next?
0 -
The basic principle is, the finer atomization exposes more of the fuel to the air, which means it can burn more cleanly. It really does work. This is one reason some larger burners use pressures up to 300 PSI.
All Steamed Up, Inc.
Towson, MD, USA
Steam, Vapor & Hot-Water Heating Specialists
Oil & Gas Burner Service
Consulting1 -
If you have not already learned this… Fuel oil in a liquid state wil not burn very easily. It all has to do with "Flash Point". In fact if you submerge a lit match in a container of fuel oil at 70°F, the fuel oil will extinguish the match.
See time stamp 6:00 of this videoSo how do we get it to burn so easily in an oil burner? You need to raise the surface of the fuel oil to the flash point. That is the temperature where the fuel oil liquid will start to evaporate. That means the fuel oil becomes a gas. Then the fuel oil gas vapor will burn easily.
Now consider a pot style burner where the only surface area is the top of the fuel in a pot. Kerosun Monitor is the only modern Pot Burner I am familiar with. There were other brands of pot burners in the 19th century and early 20th century. The atomizing burner would make those pot burners obsolete by rendering the fuel oil into small droplets. They called that process atomizing, but that name is less accurate than say dropletizing. But that does not sound as scientific as atomizing. So let's look at what exactly happens with "Atomizing" fuel oil.
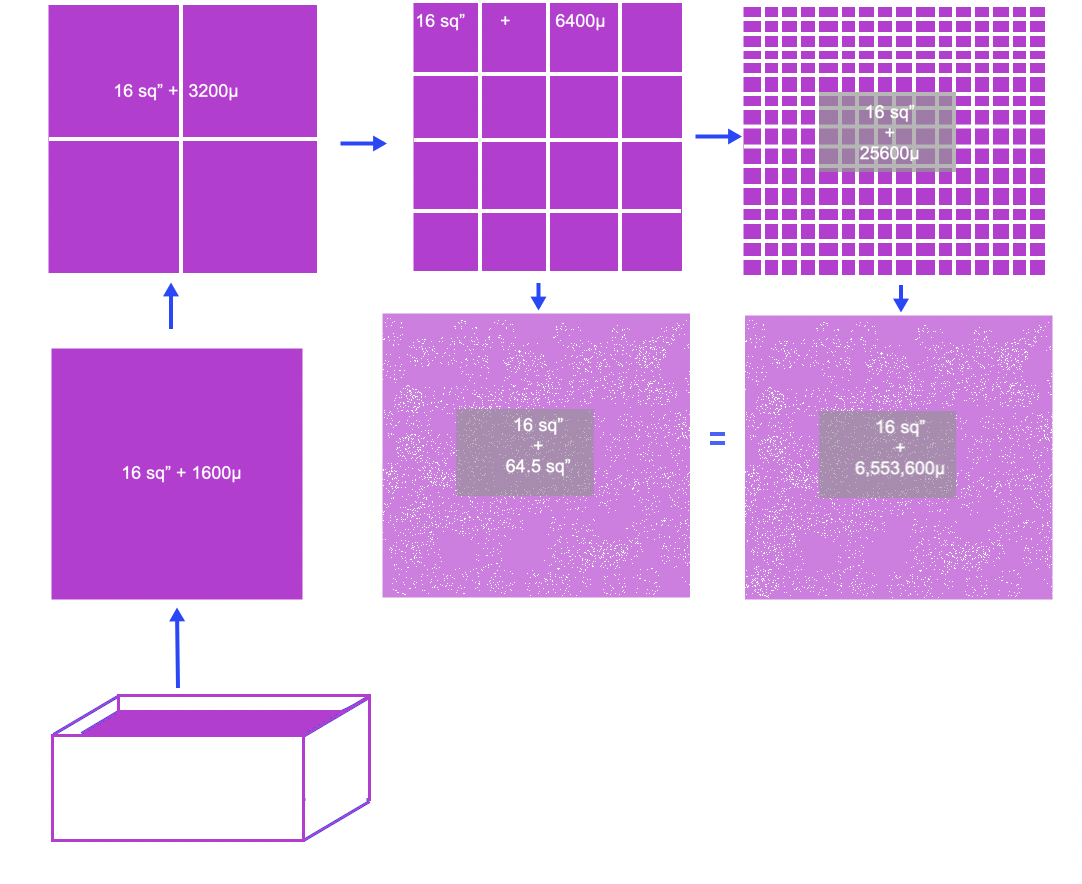
If you look at the top layer of fuel in a square pot of oil that is, say 4” x 4”. There is only 16 square inches of surface for the entire volume of fuel in the pot. If you were to take that same surface area and lay it on a flat surface only 100 micrometers thick, then there would be the 16 square inches plus the additional 16 x 100 micron (1600 µm) edge that can evaporate. (to be more specific, the oil is only 100 microns thick) 1600µm is the surface of the edge only. Let's look at that edge and cut it into 4 equal squares. That edge now goes from only 1600 microns to 32 x 100 microns 3200 µm; see the illustration. After only a few divisions like that can actually increase the 100 micron edge to have a larger surface area than the original 16sq” of the 4 x 4 surface.
Now after you absorb that concept… think about the rest of the oil in the 4x4 container that is 14” tall. That is how big the container would be to hold 1 gallon. That 14” tall container would have 3556 layers of 100 micron oil. That means that 1 gallon of oil would be converted to 229,376 sq inches of surface area at only 600 micron surface area droplets.
But that is still room temperature oil…
How do you get it hot enough to burn? Easy… with so little volume of oil behind that surface (unlike the oil in the container in the video) the spark of the ignition transformer can easily heat the small droplet in very short order. The oil in a container can absorbe the heat from the match because Heat goes to Cold. Since the oil in that container has a large volume compared to the match, there is more mass to absorb the small amount of heat provided by the kitchen match. Once the combustion process starts the heat from the flame will evaporate the droplets immediately behind the flaming fuel. It becomes self-sustaining as long as there is a consistent supply of atomized fuel.
How does higher pressure make the droplets smaller?
The higher pressure equals more potential energy. As a result of putting more energy behind the oil in the form of higher pressure, that energy causes the atomization process to accelerate in two ways. More fuel is able to go through the same size orifice and the higher energy applied to the swirling of the oil will cause the oil to break apart into smaller droplets. It is all in the physics of the surface tension of the liquid. I am no scientist, so I can't offer a dissertation on the whole Physics of liquids under pressure. All I can offer is this dumbed down version as it was explained to me by someone that knows more than me (who is probably dead now) at the time, because he understood the physics better than me. You know how that goes. Someone explains a concept to your satisfaction so you accept it as the way it is. You roll with that understanding for years and years. Then some young apprentice asks you “WHY?” and you know in the back of your mind why, but you can't form the words to explain it the way your teacher from years ago said it. Now you have to say something like “Because I said So!”
Mr. Ed
Edward Young Retired
After you make that expensive repair and you still have the same problem, What will you check next?
0 -
@BDR529 and others: If you have not already learned this… Fuel oil in a liquid state wil not burn very easily. It all has to do with "Flash Point". In fact if you submerge a lit match in a container of fuel oil at 70°F, the fuel oil will extinguish the match.
See time stamp 6:00 of this videoSo how do we get it to burn so easily in an oil burner? You need to raise the surface of the fuel oil to the flash point. That is the temperature where the fuel oil liquid will start to evaporate. That means the fuel oil becomes a gas. Then the fuel oil gas vapor will burn easily.
Now consider a pot style burner where the only surface area is the top of the fuel in a pot Kerosun Monitor is the only modern Pot Burner I am familiar with. There were other brands of pot burners in the 19th century and early 20th century. The atomizing burner would make those pot burners obsolete by rendering the fuel oil into small droplets. They called that process atomizing, but that name is less accurate than say dropletizing. But that does not sound as scientific as atomizing. So let's look at what exactly happens with "Atomizing" fuel oil.
If you look at the top layer of fuel in a square pot of oil that is, say 4” x 4”. There is only 16 square inches of surface for the entire volume of fuel in the pot. If you were to take that same surface area and lay it on a flat surface only 100 micrometers thick, then there would be the 16 square inches plus the additional 16 x 100 micron (1600 µm) edge that can evaporate. 1600µm is the surface of the edge only. Let's look at that edge and cut it into 4 equal squares. That edge now goes from only 1600 microns to 32 x 100 microns 3200 µm; see the illustration. After only a few divisions like that can actually increase the 100 micron edge to have a larger surface area than the original 16sq” of the 4 x 4 surface.
Now after you absorb that concept… think about the rest of the oil in the 4x4 container that is 14” tall. That is how big the container would be to hold 1 gallon. That 14” tall container would have 3556 layers of 100 micron oil. That means that 1 gallon of oil would be converted to 229,376 sq inches of surface area at only 600 micron surface area droplets.
But that is still room temperature oil…
How do you get it hot enough to burn? Easy… with so little volume of oil behind that surface (unlike the oil in the container in the video) the spark of the ignition transformer can easily heat the small droplet in very short order. Once the combustion process starts the heat from the flame will evaporate the droplets immediately behind the flaming fuel. It becomes self-sustaining as long as there is a consistent supply of atomized fuel.
How does higher pressure make the droplets smaller?
The higher pressure equals more potential energy. As a result of putting more energy behind the oil in the form of higher pressure, that energy causes the atomization process to accelerate in two ways. More fuel is able to go through the same size orifice and the higher energy applied to the swirling of the oil will cause the oil to break apart into smaller droplets. It is all in the physics of the surface tension of the liquid. I am no scientist, so I can't offer a dissertation on the whole Physics of liquids under pressure. All I can offer is this dumbed down version as it was explained to me by someone that knows more than me (who is probably dead now) at the time, because he understood the physics better than me. You know how that goes. Someone explains a concept to your satisfaction so you accept it as the way it is. You roll with that understanding for years and years. Then some young apprentice asks you “WHY?” and you know in the back of your mind why, but you can't form the words to explain it the way your teacher from years ago said it. Now you have to say something like “Because I said So!”
Edward Young Retired
After you make that expensive repair and you still have the same problem, What will you check next?
0 -
I would first check the underside of the porcelian for cracks . Then I would set the Riello up as per Biasi , and try using a "W" nozzle.
There was an error rendering this rich post.
0 -
You never put vanilla extract in chocolate icing. Barbaric! See your still wrong!
0
Categories
- All Categories
- 87.5K THE MAIN WALL
- 3.3K A-C, Heat Pumps & Refrigeration
- 61 Biomass
- 430 Carbon Monoxide Awareness
- 122 Chimneys & Flues
- 2.1K Domestic Hot Water
- 5.9K Gas Heating
- 116 Geothermal
- 169 Indoor-Air Quality
- 3.8K Oil Heating
- 78 Pipe Deterioration
- 1K Plumbing
- 6.6K Radiant Heating
- 395 Solar
- 15.9K Strictly Steam
- 3.5K Thermostats and Controls
- 57 Water Quality
- 51 Industry Classes
- 51 Job Opportunities
- 18 Recall Announcements