Welcome! Here are the website rules, as well as some tips for using this forum.
Need to contact us? Visit https://heatinghelp.com/contact-us/.
Click here to Find a Contractor in your area.
If our community has helped you, please consider making a contribution to support this website. Thanks!
Test question from today:
Options
Eric_32
Member Posts: 267
Took the Pipefitter license test today for RI, and got this question that had me thinking for while:
<strong>Is it more efficient to heat hot water using a steam boiler or hot water boiler?</strong>
Efficiencies and sizes were not given, so I am asuming both boilers would be the exact same brand and size, similar except for the duration they heat the water, hot water to 180°, and steam off the pressuretrol.
I didn't know if this references potable water as in a tankless coil, or a hot water zone which could be added to a steam boiler just like a water filled boiler.
I put they would be similar in efficiency as my answer thinking the steam boiler and water boiler could both be heated by the same model burner and would heat the water at the same rate getting water to the same temperature the same time, all happening before water got to boil.
Anyone know this and would like to share their answer?
thanks!!
<strong>Is it more efficient to heat hot water using a steam boiler or hot water boiler?</strong>
Efficiencies and sizes were not given, so I am asuming both boilers would be the exact same brand and size, similar except for the duration they heat the water, hot water to 180°, and steam off the pressuretrol.
I didn't know if this references potable water as in a tankless coil, or a hot water zone which could be added to a steam boiler just like a water filled boiler.
I put they would be similar in efficiency as my answer thinking the steam boiler and water boiler could both be heated by the same model burner and would heat the water at the same rate getting water to the same temperature the same time, all happening before water got to boil.
Anyone know this and would like to share their answer?
thanks!!
0
Comments
-
Answer?
I would say that there are more than one "right" answer.
IMO, if it is a hot water boiler is a hot water boiler, and you raise the water temperature to a point where you can make hot water but not steam, the hot water is more efficient. But if it is a steam boiler, and you are using s steam coil, and you have to run steam through it to make the hot water, the hot water boiler would definitely be more efficient.
I'm running out of battery power. I'll have to see the answer later.0 -
Tough Question
That question sucks."Is it more efficient to heat hot water using a steam boiler or hot water boiler? "
1. Why are we trying to heat "hot" water? Are we trying to make it hotter? Are we trying to make steam?
2. If we are talking apple to apple boilers, they will both run at the same efficiency until the hot water boiler shuts of at 211 degrees and the steam boiler runs on. That would make the hot water boiler more efficient as it would use less fuel?
3. If we are talking a mod con vs. cast iron steam its a no brainer.
4. Are they talking domestic through a coil?
I'm getting overwhelmed at all the different possibilities of such an open ended question. Lets us know the answer and the reasoning if you ever find out. I'm putting my money on the hot water boiler just because the schools don't push steam anymore.
Rob0 -
Given the framework
A BTU is a BTU.0 -
Asked my co workers this....
I asked my co workers this and one's response was:
Steam boiler would be as it is at 0 PSI and the water boiler is at say 12-15 PSI. As pressure increases so does boiling point, so he feels that formula is the same as water is heated up as well, which I don't actually know if that is true. (Does water under pressure take longer to reach a certain temp than at atmospheric pressure)?
Another mentioned boiler for boiler, side by side, the identical steam boiler would hold less water that the water filled boiler, so it would heat faster, therefore being more efficient.
Well I will find out in a couple of days, if I pass, I may never know. If if fail they will give me the wrong ones I think.0 -
translation
It's a bad question.
They're unfortunately common on licensing exams...0 -
Hot water, because
the steam system requires the btu's to heat the water and the additional energy to change water to steam. A phase change takes energy.
So it must be more efficient to just heat the water.
Think of it this way, if you built a perfectly insulated box around both systems, the hot water would be perfectly efficient to move the energy created by the boiler to the hot water tank. But the steam system would lose the energy until it was released when the steam condensed somewhere in the steam pipes.0 -
The Question
"Is it more efficient to heat HOT WATER using a steam boiler or hot water boiler"?0 -
how do you make hot water from a steam boiler
without making steam?0 -
heat of vaporization
Heat of Vaporization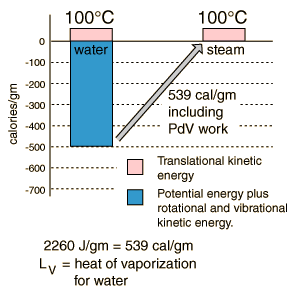
The energy required to change a gram of a liquid into the gaseous state at the boiling point
is called the "heat of vaporization". This energy breaks down the
intermolecular attractive forces, and also must provide the energy
necessary to expand the gas (the PDV work). For an ideal gas , there is no longer any potential energy associated with intermolecular forces. So the internal energy is entirely in the molecular kinetic energy.
The final energy is depicted here as being in translational kinetic
energy, which is not strictly true. There is also some vibrational and
rotational energy.
A significant feature of the vaporization phase change of water is the large change in volume that accompanies it. A mole of water is 18 grams, and at STP
that mole would occupy 22.4 liters if vaporized into a gas. If the
change is from water to steam at 100°C, rather than 0°C, then by the ideal gas law that volume is increased by the ratio of the absolute temperatures,
373K/273K, to 30.6 liters. Comparing that to the volume of the liquid
water, the volume expands by a factor of 30600/18 = 1700 when vaporized
into steam at 100°C. This is a physical fact that firefighters know,
because the 1700-fold increase in volume when water is sprayed on a fire
or hot surface can be explosive and dangerous.0 -
Over thinking it
Maybe. If that is exactly how the question was worded the water to be heated is already hot.
Kinda like the question: If you dig a hole 6'x6'x6' how many cubic yards of material is in the hole? Answer: 0. There is nothing in the hole.
So I agree with Paul48
Gordy0 -
I'll answer with a ?
How do you make steam in a steam boiler without heating the water?0 -
I'd say hot water
Assuming the hot water we are heating is DHW, then a further reasonable assumption is the load varries, so the boiler cycles. Hot water would win.There was an error rendering this rich post.
0 -
Not enough info given
To give an accurate, and complete answer. Like Paul said a Btu is a Btu.
The question does not indicate if the water is domestic, or boiler content water. Nore does it indicate what temp the water is, and to what temp the water will be heated to, or what pressure the water to be heated is.
All in all a Btu is a Btu. If boiler efficiency is identical then they should be the same.0 -
I agree with Paul too
a btu is a btu. Putting it another way, energy is neither created nor destroyed.
The first law of thermodynamics:
First law of thermodynamics: Heat and work are forms of energy transfer. Energy is invariably conserved but the internal energy of a closed system changes as heat and work are transferred in or out of it. Equivalently, perpetual motion machines of the first kind are impossible.
So, for this question, which obviously leaves out details on systems, but only mentions water and steam as ways to heat water, they would be identically efficient until the steam system made steam. At that point any heat of vaporization became "friction" in the system and is energy transferred away from the "task at hand" of heating the hot water.
Asking the question another way, if they are equally efficient, where did the energy come from to vaporize the water to make steam?0 -
BTU
A British Thermal Unit (BTU) is the amount of heat energy needed to raise the temperature of one pound of water by one degree F.
Now take an identical weight and temperature water in both types of boilers. It will take the same btus to raise the temperature of that water(and still have it be water), therefore it will have used the same amount of energy. The efficiency is the same.0 -
off topic - but I love VictoriaEnergy's signature line
Not a heating pro by any means - I come here to learn, and to share when I have experience that is relevant.
My day job is computer - electronics - video troubleshooter - and I agree wholeheartedly with your tag line:
As troubleshooters, we often find out that we've missed - wouldn't it be great to get feedback when we hit the bullseye?
Vbob0
This discussion has been closed.
Categories
- All Categories
- 87.3K THE MAIN WALL
- 3.2K A-C, Heat Pumps & Refrigeration
- 61 Biomass
- 429 Carbon Monoxide Awareness
- 120 Chimneys & Flues
- 2.1K Domestic Hot Water
- 5.8K Gas Heating
- 115 Geothermal
- 166 Indoor-Air Quality
- 3.7K Oil Heating
- 77 Pipe Deterioration
- 1K Plumbing
- 6.5K Radiant Heating
- 395 Solar
- 15.7K Strictly Steam
- 3.4K Thermostats and Controls
- 56 Water Quality
- 51 Industry Classes
- 50 Job Opportunities
- 18 Recall Announcements


