Haloecetic Acids, Trimethylhalides, Lions, and Tigers, and Bears. Oh my.

Comments
-
I'm still trying to learn how to properly treat a steam boiler. I've been using Steamaster tablets since 2012 with excellent results but I'm trying to learn more about it as we speak.
If I was you, I would contact Rhomar Water Managment and ask them for recommendations. They sell test kits, treatment, everything you need.
http://www.rhomarwater.com/
Give them a call, explain the concern and see what they recommend. You can't go wrong.Single pipe quasi-vapor system. Typical operating pressure 0.14 - 0.43 oz. EcoSteam ES-20 Advanced Control for Residential Steam boilers. Rectorseal Steamaster water treatment0 -
I tested a 1 cup sample of my boiler water yesterday with my new digital pH meter and got a value of 7.6. I had gotten around 6.6 when I was trying to use the pH hydrion paper but didn't believe it was accurate. Anyway, I then proceeded to add about 0.010 oz of caustic soda and the pH shot all the way up to 12.3 !!! I think I need a new digital scale that is more accurate with small weights like that because it was hard to get a stable reading. It seems like 1/4 tsp of the caustic soda was about .050 oz, so that would have been about 1/20 tsp that I put in.
So now I'm trying to decide how much caustic soda I would need to add to my 10.8 gal boiler to get pH 10.2. Since pH is logarithmic, I believe this was about 501x too much caustic soda:
10^(12.3-7.6)/10^(10.2-7.6) = 501
I can't possibly measure out 0.010 oz/501 = 1.996e^-5 oz to test this on another 1 cup sample from the boiler.
But......I would need to have 10.8 gal x 16 cups/gal x 0.010 oz/501 = 0.00345 oz of caustic soda in the boiler. Still too small for me to measure out.
So I believe that I would need to take a 2.5 gal pail of water and put 40 x 0.00345 oz = .138 oz of caustic soda in it and mix it up and draw off 1 cup and pour that into the boiler. That amount I can measure accurately with my scale, but my original 0.010 oz may be in error.
Did I figure this out correctly? Sheesh, I can see why people probably just do trial and error.
0 -
I just learned that both sodium nitrite and sodium sulphite both apparently work as oxygen scavengers.
Single pipe quasi-vapor system. Typical operating pressure 0.14 - 0.43 oz. EcoSteam ES-20 Advanced Control for Residential Steam boilers. Rectorseal Steamaster water treatment0 -
Just a few questions, to see if I can help. How did you measure the pH of the soda? How long did the solution mix in your boiler before you grabbed a sample? Where is your sampling port in relation to the addition port you used? Does your pH meter readout adjust based on the temp of the solution? You're on the right track if you plan on adding a solution instead of a powder, it will make your life much easier.Captain Who said:I tested a 1 cup sample of my boiler water yesterday with my new digital pH meter and got a value of 7.6. I had gotten around 6.6 when I was trying to use the pH hydrion paper but didn't believe it was accurate. Anyway, I then proceeded to add about 0.010 oz of caustic soda and the pH shot all the way up to 12.3 !!! I think I need a new digital scale that is more accurate with small weights like that because it was hard to get a stable reading. It seems like 1/4 tsp of the caustic soda was about .050 oz, so that would have been about 1/20 tsp that I put in.
So now I'm trying to decide how much caustic soda I would need to add to my 10.8 gal boiler to get pH 10.2. Since pH is logarithmic, I believe this was about 501x too much caustic soda:
10^(12.3-7.6)/10^(10.2-7.6) = 501
I can't possibly measure out 0.010 oz/501 = 1.996e^-5 oz to test this on another 1 cup sample from the boiler.
But......I would need to have 10.8 gal x 16 cups/gal x 0.010 oz/501 = 0.00345 oz of caustic soda in the boiler. Still too small for me to measure out.
So I believe that I would need to take a 2.5 gal pail of water and put 40 x 0.00345 oz = .138 oz of caustic soda in it and mix it up and draw off 1 cup and pour that into the boiler. That amount I can measure accurately with my scale, but my original 0.010 oz may be in error.
Did I figure this out correctly? Sheesh, I can see why people probably just do trial and error.
You can have it good, fast or cheap. Pick two0 -
I haven't put any caustic soda in the boiler yet. I just took a sample of the boiler water before attempting to adjust its pH. I took the sample and added 0.010 oz (plus or minus whatever error) of caustic soda to it and got way too high of a pH of 12.3. I am using this to try to calculate how much caustic soda my boiler will need added to it to give a pH of 10.2.
The sampling port was the bottom of the sight glass. I had quite a bit of sediment in it, which after it settled overnight to the bottom of the 1 cup pyrex measure was about 1/4" (wow). I had some plastic wrap on the top with a rubber band to reduce absorption of carbon dioxide. The pH meter was calibrated to a test solution at room temperature and then used to test the boiler sample. There is a table of pH for the test solution so you know what it should be based upon the testing temperature.
I'll probably have to remove the pressure relief valve and pour the caustic soda and water solution in through there.
0 -
If my math is right this morning, you have an 11% caustic solution in your cup(I'm assuming 250mL) I'm not sure how you're going to ensure the solution in your boiler ends up thoroughly mixed though.(I have a mixer or circulation pump in the vessels I have to pH where I work. Plus I have access to nitrogen gas I can bubble through the solution if the unit has neither, I doubt you want to bubble air through your boiler.)
If it was me, I would make a similar percent solution in a pail and use that to adjust your pH, should mix fairly easily when it steams. If you make 10 L of solution be conservative when adding it to the boiler,(2 oz or less to start) as you found out from your beaker, it doesn't take much to adjust if there isn't much to react with. Keep a log of your additions, (temp of fluid and amount of solution added should be sufficient) It will help if the boiler water is buffering while your adjusting pH, which could result in you overshooting your pH target.
This is going to be slow unless you have a way to mix your boiler water, so be patient.You can have it good, fast or cheap. Pick two0 -
There are many buffer reactions which will take place as you are trying to adjust your pH. Unless you know the complete chemical composition of the water, you can't assume a linear or logarithmic relationship between adding a strong base -- your caustic soda -- and pH. As Canucker noted, this is tricky -- even in the lab.Br. Jamie, osb
Building superintendent/caretaker, 7200 sq. ft. historic house museum with dependencies in New England1 -
@ Canucker and Jamie Hall - thanks for the input. I think I'll only put half a cup of the solution with my calculated amount (drawn off of the 2.5gal master sol'n) of NaOH in 1 cup of distilled water. I'll wash it down with 1/2 cup of distilled water. Then I'll wait a week and draw another sample of boiler water and test the pH. If it looks like I'm on target, ie. I get a pH of 9.9 I'll assume it is OK to put in the other half. If not, I'll reassess the situation.
7.6 + log (0.50 x 10^(10.2-7.6)) = 9.90 -
Sounds like a good plan. Definitely keep track of the final pH after an addition, you'll need that to see if it's buffering. I forgot to mention that in my earlier post.(The log is useless without a final pH. lol) Keeping a log will definitely help you get a feel for how much to add to your boiler in the future. Like Jamie said, you never know the complete story of the solution because you don't test for everything that will affect it. Good luckYou can have it good, fast or cheap. Pick two0
-
Holy cow, chemistry.0
-
Yeah,AnnieT said:Holy cow, chemistry.
Relax and call http://www.rhomarwater.com/. They will tell you what you need to do.
This stuff is making my head hurt too. Single pipe quasi-vapor system. Typical operating pressure 0.14 - 0.43 oz. EcoSteam ES-20 Advanced Control for Residential Steam boilers. Rectorseal Steamaster water treatment0
Single pipe quasi-vapor system. Typical operating pressure 0.14 - 0.43 oz. EcoSteam ES-20 Advanced Control for Residential Steam boilers. Rectorseal Steamaster water treatment0 -
Thanks for the tip. A premix...interesting.
I wonder how many water and sewer pipes are affected by changing levels of chlorine and the funky bunch mentioned above in municipal supplies.0 -
Also, can I use pH strips like the kind that measure body pH levels to test the boiler water? Before I call, I know I'm going to need a baseline read. Those fancy digital pH meters are $$0
-
Depends on the range.AnnieT said:Also, can I use pH strips like the kind that measure body pH levels to test the boiler water? Before I call, I know I'm going to need a baseline read. Those fancy digital pH meters are $$
I've been using pHydrion strips that do 0 - 13 I think. Going to be switching to much more narrow ones soon that I think are 6-12 or something like that.
If you buy general purpose pHydrion ones, they should work.Single pipe quasi-vapor system. Typical operating pressure 0.14 - 0.43 oz. EcoSteam ES-20 Advanced Control for Residential Steam boilers. Rectorseal Steamaster water treatment0 -
I have 0-13 and 5.5-8.0. It was only useful for a very rough estimate. I also have a Dr. Meter® PH001 High Accuracy Pocket Size pH Meter with ATC (Automatic Temperature Compensation) Backlit Light LCD 0-14 pH Measurement Range, 0.01 Resolution Handheld pH Pen Tester. Too soon to tell if it is a high quality unit or not. Seems pretty touchy as far as calibration goes and needs to be calibrated every time I think, even though I am trying to keep the electrode wet in calibration fluid.
I just re-tested my boiler water sample that I got the reading of 12.3 with the digital meter, with the 0-13 I guessed 12 but I really couldn't tell for sure that it wasn't 13. That's not good enough because 13 is 10x more alkaline than 12.
@ChrisJ - If you find the 6-12 hydrion paper please let us know where to get it. That would be ideal as a more convenient backup for the digital meter. I have found 6-8, 8-9.5, and 10-12 but so far they are only a part of a $45 set of 6 different ranges. That would work pretty well for convenience, but still you might need a backup digital, especially if your water is full of fine brown sediment and you can't wait for it to settle.0 -
These are the ones I ordered. Had to order 5 minimum direct from manufacturer because they are hard to find. I liked them because 9 is very obvious.Captain Who said:I have 0-13 and 5.5-8.0. It was only useful for a very rough estimate. I also have a Dr. Meter® PH001 High Accuracy Pocket Size pH Meter with ATC (Automatic Temperature Compensation) Backlit Light LCD 0-14 pH Measurement Range, 0.01 Resolution Handheld pH Pen Tester. Too soon to tell if it is a high quality unit or not. Seems pretty touchy as far as calibration goes and needs to be calibrated every time I think, even though I am trying to keep the electrode wet in calibration fluid.
I just re-tested my boiler water sample that I got the reading of 12.3 with the digital meter, with the 0-13 I guessed 12 but I really couldn't tell for sure that it wasn't 13. That's not good enough because 13 is 10x more alkaline than 12.
@ChrisJ - If you find the 6-12 hydrion paper please let us know where to get it. That would be ideal as a more convenient backup for the digital meter.
6-11
https://www.microessentiallab.com/ProductInfo/F01-WIDRG-060110-SRD.aspxSingle pipe quasi-vapor system. Typical operating pressure 0.14 - 0.43 oz. EcoSteam ES-20 Advanced Control for Residential Steam boilers. Rectorseal Steamaster water treatment0 -
Thank you for the help!0
-
This looks good for when you are "zeroing in on it", after trial and error adjustments:
Ph Hydrion Papers 9.2-10.6 - Great for Testing Soap Solutions
http://www.amazon.com/Ph-Hydrion-Papers-9-2-10-6-Solutions/dp/B0075WH5A4/ref=sr_1_42?ie=UTF8&qid=1421078109&sr=8-42&keywords=ph+hydrion
This could be good for rough measurements:
pH Strips, Hydrion Spectral, 6.5-13, PK 100
http://www.amazon.com/Strips-Hydrion-Spectral-6-5-13-100/dp/B0045I6KHU/ref=sr_1_91?ie=UTF8&qid=1421078659&sr=8-91&keywords=ph+hydrion0 -
If you can keep your electrode in a storage solution, you should be able to cut down on the times you need to calibrate. Keep particulate from getting stuck to the electrode, dirty ones can give really bad readings. If you clean and calibrate for every pH session, you should be fine.Captain Who said:I have 0-13 and 5.5-8.0. It was only useful for a very rough estimate. I also have a Dr. Meter® PH001 High Accuracy Pocket Size pH Meter with ATC (Automatic Temperature Compensation) Backlit Light LCD 0-14 pH Measurement Range, 0.01 Resolution Handheld pH Pen Tester. Too soon to tell if it is a high quality unit or not. Seems pretty touchy as far as calibration goes and needs to be calibrated every time I think, even though I am trying to keep the electrode wet in calibration fluid.
You can have it good, fast or cheap. Pick two0 -
Do you think it's that critical?Captain Who said:This looks good for when you are "zeroing in on it", after trial and error adjustments:
Ph Hydrion Papers 9.2-10.6 - Great for Testing Soap Solutions
http://www.amazon.com/Ph-Hydrion-Papers-9-2-10-6-Solutions/dp/B0075WH5A4/ref=sr_1_42?ie=UTF8&qid=1421078109&sr=8-42&keywords=ph+hydrion
This could be good for rough measurements:
pH Strips, Hydrion Spectral, 6.5-13, PK 100
http://www.amazon.com/Strips-Hydrion-Spectral-6-5-13-100/dp/B0045I6KHU/ref=sr_1_91?ie=UTF8&qid=1421078659&sr=8-91&keywords=ph+hydrion
My opinion is anything 9-10 should be perfectly fine, no?Single pipe quasi-vapor system. Typical operating pressure 0.14 - 0.43 oz. EcoSteam ES-20 Advanced Control for Residential Steam boilers. Rectorseal Steamaster water treatment0 -
@ChrisJ - I don't really know. The article I posted previously said 10.2 is optimal for corrosion protection. Maybe it will foam too much at that? While I'm getting a handle on it, I prefer to err on the side of too much accuracy. I want to test periodically to see if it changes over time, like maybe every 4 weeks.
@Canucker - I've been keeping it in the storage cap which has some of the pH 7.0 calibration solution in it. Is that OK, or do you have to buy a so called "storage solution"? What's the difference?0 -
I think you'll need something slightly more acidic. Don't store it in distilled or deionized water.This is what I'm familiar with usingCaptain Who said:@ChrisJ - I don't really know. The article I posted previously said 10.2 is optimal for corrosion protection. Maybe it will foam too much at that? While I'm getting a handle on it, I prefer to err on the side of too much accuracy. I want to test periodically to see if it changes over time, like maybe every 4 weeks.
@Canucker - I've been keeping it in the storage cap which has some of the pH 7.0 calibration solution in it. Is that OK, or do you have to buy a so called "storage solution"? What's the difference?
http://www.fondriest.com/thermo-orion-ph-electrode-storage-solution.htm
I'd use the pH 4 calibration solution, if I couldn't get storage solution. 7 will work for short storage periods
You can have it good, fast or cheap. Pick two0 -
@Canucker - Thanks. I have the 4.0 pH calibration solution as well but I was trying to cut corners thinking that if I store it in the 7.0, which is where I've been calibrating it since it is closer to my sample measuring pH, I could calibrate and store it at the same time, if you get my drift. What is the disadvantage of storing it at a higher pH?
Also, I've always wondered what is the difference between distilled water and deionized? My distilled water is very acidic due to carbonic acid (I guess).0 -
@chrisj I just checked my PH using a kit that goes from ph 1-14 and it looks like the ph is 10. I did just order the ph 6 to 11 strip you mentioned, it will be interesting to see if the two agree.
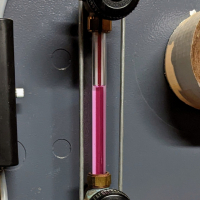
The water has a very light violet hue (Steam master added over 2 years ago), The water I drained from the mud leg was clean) I use one tablet for the Smith G8-3 boiler and I actually dumped a couple of gallons initially because I thought the ph too high when I started out.
BobSmith G8-3 with EZ Gas @ 90,000 BTU, Single pipe steam
Vaporstat with a 12oz cut-out and 4oz cut-in
3PSI gauge0 -
Distilled water is less "pure", deionized is RO-ized? hmmm...here's a link0
-
What is the pH of your tap water? Mine is 7.BobC said:@chrisj I just checked my PH using a kit that goes from ph 1-14 and it looks like the ph is 10. I did just order the ph 6 to 11 strip you mentioned, it will be interesting to see if the two agree.
The water has a very light violet hue (Steam master added over 2 years ago), The water I drained from the mud leg was clean) I use one tablet for the Smith G8-3 boiler and I actually dumped a couple of gallons initially because I thought the ph too high when I started out.
BobSingle pipe quasi-vapor system. Typical operating pressure 0.14 - 0.43 oz. EcoSteam ES-20 Advanced Control for Residential Steam boilers. Rectorseal Steamaster water treatment0 -
-
@BobC - If your water has a light violet hue from the Steammaster tablets, do you think that might throw off the pH paper method, since it uses a color chart?0
-
This is a really simplified answer but Deionized water uses a resin bed to remove its impurities while distilled water comes from condensate, usually leaving the impurites in the pot you are boiling. As usual, when it comes to water and the method you use, the difference depends on a few factors. Here's a pretty good explanation on why and how to store a meter.
http://bitesizebio.com/8750/how-to-care-for-your-ph-meter/
You can have it good, fast or cheap. Pick two0 -
The hue is so light I don't think it's affecting the test. The fact the boiler water is still clean after a couple of years makes me happy. We'll see if the new strips agree.
My tap water is 7 as far as i can tell.
BobSmith G8-3 with EZ Gas @ 90,000 BTU, Single pipe steam
Vaporstat with a 12oz cut-out and 4oz cut-in
3PSI gauge0 -
My concern with this is we have no way of knowing what your nitrite levels are.BobC said:The hue is so light I don't think it's affecting the test. The fact the boiler water is still clean after a couple of years makes me happy. We'll see if the new strips agree.
My tap water is 7 as far as i can tell.
Bob
Sodium nitrite is the corrosion inhibitor in Steamaster and apparently it also works as an oxygen scavenger.
This gets used up over time and has no effect on the violet color. I change my water typically once a year and give a good washing using the wand so I end up with two fresh tablets in every year.Single pipe quasi-vapor system. Typical operating pressure 0.14 - 0.43 oz. EcoSteam ES-20 Advanced Control for Residential Steam boilers. Rectorseal Steamaster water treatment0 -
I'm thinking about buying this to use in addition to Steamaster.
http://www.amazon.com/Nitrite-Granular-Flowing-Processing-Manufacturing/dp/B00L74HO5M/ref=sr_1_1?ie=UTF8&qid=1421084271&sr=8-1&keywords=sodium+nitriteSingle pipe quasi-vapor system. Typical operating pressure 0.14 - 0.43 oz. EcoSteam ES-20 Advanced Control for Residential Steam boilers. Rectorseal Steamaster water treatment0 -
true.0
-
if municipalities treated the water with UV radiation, it would be more effective at killing off e-coli and other nasties and would be gentler on plumbing and steam boilers. Chloramine (Chlorine plus ammonia) is a hazard to health. frightening.0
-
Ozone and/or UV are quite effective at killing critters. Required doses change day by day, particularly for surface water. When you turn on the shower and get a strong chlorine smell, it generally indicates there was bloom of some sort in the reservoir. Standard practice in most of the EU (and a few lucky places here in the US) is to dose as required with ozone, then an ozone destruct stage (ozone eats metal pipes) followed by a tiny dose of chlorine or chlorine dioxide to protect the water in the distribution system. This results in far lower residual Cl2 levels than most of us are used to. Chloramine does an even better job of persisting in the distribution system, but mixes badly with copper.0
-
And then there's the problem of critters in the pipes, far away from the treatment facility. The anti-chloramine websites I've looked provide some links to studies that show it's ineffectiveness at killing e-coli and other pathogens. Too bad infrastructure wasn't considered more carefully before building booms. Consider the flooded towns that are now the Quabbin Reservoir in central MA. A hearty percent of that water is lost in transmission. But Boston needs its water.
0 -
All this boiler chemistry for residential is a bit overkill. But if you are that involved I would do chloride tests and hardness top of the pH. each of these tests can be purchased from Hach. These tests actually test for the contaminant divalent salts (any 2+ like calcium and magnesium) that scale and if the temp&psi is right chlorides that acidify the boiler. this kind of exactness will require maintenance intensive makeup and then blow down to control cyclic concentration. At this temp and pressure I guess the limits are slightly above .5 mg/l of hardness and probably around 300ppm chloride. Are you guys seeing such a benefit for 1lb boiler operation? If you are looking for standard chemicals to treat your water without any proprietary products I would suggest finding old us navy or marine engineer books on boiler treatment. They are the ones that figured out real quick that as they changed pressure requirements of marine boilers saltwater wasn't an option. They even will give you the ph then titration test for chloride to be done with standard available chemicals. Traditional boiler chemistry was founded and written by the U.S. navy and marine engineers. The 1920's to 30's books will deal with lower pressures and can be used in application to residential I imagine. after the 40's pressures went way up in boiler tech and the chemistry gets real tight. the books are all over ebay for less than 10 bucks.
Since most of us don't purify our water to house boilers, phosphate treatment seems to be a viable choice. if you did purify your water then caustic alone would be good for pH control.
If you want to start testing makeup water for chlorine you can purchase an ORP meter. like the glass type pH meters ORP meters need frequent calibrations and proper storage.0 -
Larry said:
All this boiler chemistry for residential is a bit overkill. But if you are that involved I would do chloride tests and hardness top of the pH. each of these tests can be purchased from Hach. These tests actually test for the contaminant divalent salts (any 2+ like calcium and magnesium) that scale and if the temp&psi is right chlorides that acidify the boiler. this kind of exactness will require maintenance intensive makeup and then blow down to control cyclic concentration. At this temp and pressure I guess the limits are slightly above .5 mg/l of hardness and probably around 300ppm chloride. Are you guys seeing such a benefit for 1lb boiler operation? If you are looking for standard chemicals to treat your water without any proprietary products I would suggest finding old us navy or marine engineer books on boiler treatment. They are the ones that figured out real quick that as they changed pressure requirements of marine boilers saltwater wasn't an option. They even will give you the ph then titration test for chloride to be done with standard available chemicals. Traditional boiler chemistry was founded and written by the U.S. navy and marine engineers. The 1920's to 30's books will deal with lower pressures and can be used in application to residential I imagine. after the 40's pressures went way up in boiler tech and the chemistry gets real tight. the books are all over ebay for less than 10 bucks.
Since most of us don't purify our water to house boilers, phosphate treatment seems to be a viable choice. if you did purify your water then caustic alone would be good for pH control.
If you want to start testing makeup water for chlorine you can purchase an ORP meter. like the glass type pH meters ORP meters need frequent calibrations and proper storage.
With all due respect with so many residential steam boilers rotting out every 3-10 years I don't think it's a bit overkill. If on average a modern boiler was lasting 30 years, maybe, but that's not the case.Single pipe quasi-vapor system. Typical operating pressure 0.14 - 0.43 oz. EcoSteam ES-20 Advanced Control for Residential Steam boilers. Rectorseal Steamaster water treatment0 -
I discovered this link which has a great deal of information:
GE Power & Water/ Water & Process Technologies
Handbook of Industrial Water Treatment
http://www.gewater.com/handbook/index.jsp1
Categories
- All Categories
- 85.7K THE MAIN WALL
- 3K A-C, Heat Pumps & Refrigeration
- 49 Biomass
- 418 Carbon Monoxide Awareness
- 72 Chimneys & Flues
- 1.9K Domestic Hot Water
- 5.2K Gas Heating
- 92 Geothermal
- 149 Indoor-Air Quality
- 3.2K Oil Heating
- 59 Pipe Deterioration
- 850 Plumbing
- 5.8K Radiant Heating
- 372 Solar
- 14.5K Strictly Steam
- 3.2K Thermostats and Controls
- 58 Water Quality
- 39 Industry Classes
- 47 Job Opportunities
- 15 Recall Announcements